RADIOACTIVITY
The Nucleus of an Atom
The Structure of the Nucleus of an Atom
Describe the structure of the nucleus of an atom
The word atom is derived from the Greek word 'atomos' which means indivisible. The Greeks concluded that matter could be broken down into particles too small to be seen. These particles were called atoms.
Atoms are composed of three type of particles: protons, neutrons, and electrons. Protons and neutrons are responsible for most of the atomic mass. The mass of an electron is very small (9.108 X 10-28 grams).
Both the protons and neutrons reside in the nucleus. Protons have a positive (+) charge, neutrons have no charge i.e they are neutral. Electrons reside in orbitals around the nucleus. They have a negative charge (-).
It is the number of protons that determines the atomic number, e.g., H = 1. The number of protons in an element is constant (e.g., H=1, Ur=92) but neutron number may vary, so mass number (protons + neutrons) may vary.
The same element may contain varying numbers of neutrons; these forms of an element are called isotopes. The chemical properties of isotopes are the same, although the physical properties of some isotopes may be different.
The Atomic Number, Mass Number and Isotopes of an Element and their Symbols
Explain the atomic number, mass number and isotopes of an element and their symbols
The atomic number of a chemical element (also known as its proton number) is the number of protons found in the nucleus of an atom of that element.Therefore it is identical to the charge number of the nucleus. It is conventionally represented by the symbol Z.
The atomic number uniquely identifies a chemical element. In an uncharged atom, the atomic number is also equal to the number of electrons.
The atomic number, Z, should not be confused with the mass number, A.
Mass number is the number of nucleons, i. e the total number of protons and neutrons in the nucleus of an atom. ---The number of neutrons, N, is known as the neutron number of the atom; thus, A = Z + N (these quantities are always whole numbers).
Since protons and neutrons have approximately the same mass (and the mass of the electrons is negligible for many purposes) and the mass defect of nucleon binding is always small compared to the nucleon mass, the atomic mass of any atom, when expressed in unified atomic mass units (making a quantity called the "relative isotopic mass"), is roughly (to within 1%) equal to the whole number A.
Isotopes
Isotopes are atoms with the same atomic number Z but different neutron numbers N, and hence different atomic masses.
A little more than three-quarters of naturally occurring elements exist as a mixture of isotopes (see monoisotopic elements), and the average isotopic mass of an isotopic mixture for an element (called the relative atomic mass) in a defined environment on Earth, determines the element's standard atomic weight.
Historically, it was these atomic weights of elements (in comparison to hydrogen) that were the quantities measurable by chemists in the 19th century.The chemical properties of isotopes are the same, although the physical properties of some isotopes may be different.
Some isotopes are radioactive-meaning they "radiate" energy as they decay to a more stable form, perhaps another element half-life: time required for half of the atoms of an element to decay into stable form. Another example is oxygen, with atomic number of 8 can have 8, 9, or 10 neutrons.
Forces Holding the Nucleus
Mention forces holding the nucleus
Stable and unstable atoms
There are forces within the atom that account for the behavior of the protons, neutrons, and electrons. Without these forces, an atom could not stay together.
Recall that protons have a positive charge, electrons a negative charge, and neutrons are neutral. According to the laws of physics, like charges repel each other and unlike charges attract each other. A force called the strong force opposes and overcomes the force of repulsion between the protons and holds the nucleus together.
The net energy associated with the balance of the strong force and the force of repulsion is called the binding energy. The electrons are kept in orbit around the nucleus because there is an electromagnetic field of attraction between the positive charge of the protons and the negative charge of the electrons.
In some atoms, the binding energy is great enough to hold the nucleus together. The nucleus of this kind of atom is said to be stable. In some atoms the binding energy is not strong enough to hold the nucleus together, and the nuclei of these atoms are said to be unstable. Unstable atoms will lose neutrons and protons as they attempt to become stable.
- Binding energy is the net energy that is the result of the balance with the strong force and the repulsive force, and this is the amount of energy that holds the nucleus together.
- A stable atom is an atom that has enough binding energy to hold the nucleus together permanently.
- An unstable atom does not have enough binding energy to hold the nucleus together permanently and is called a radioactive atom.
Natural Radioactivity
The Concept of Radioactivity
Explain the concept of radioactivity
Radioactive decay, also known as nuclear decay or radioactivity, is the process by which a nucleus of an unstable atom loses energy by emitting ionising radiation.
A material that spontaneously emits such radiation (alpha particles, beta particles, gamma rays and conversion electrons) is considered radioactive.
Radioactive decay is a stochastic (i.e. random) process at the level of single atoms, in that, according to quantum theory, it is impossible to predict when a particular atom will decay.
The chance that a given atom will decay never changes, that is, it does not matter how long the atom has existed. For a large collection of atoms however, the decay rate for that collection can be calculated from their measured decay constants or half-lives. The half-lives of radioactive atoms have no known limits for shortness or length of duration.
Properties of the Radiations Emitted by Radio-active Substances
Describe properties of the radiations emitted by radio-active substances
There are many types of radioactive decay. A decay, or loss of energy, results when an atom with one type of nucleus, called the parent radionuclide (or parent radioisotope), transforms into an atom with a nucleus in a different state, or with a nucleus containing a different number of protons and neutrons. The product is called the daughter nuclide. In some decays, the parent and the daughter nuclides are different chemical elements, and thus the decay process results in the creation of an atom of a different element. This is known as a nuclear transmutation.
The Nuclear Changes due to the Emission of Alpha α, Betaβ and Gammaγ Radiations
Explain the nuclear changes due to the emission of Alpha α, Betaβ and Gammaγ radiations
Properties of Alpha Rays
- Alpha rays or alpha particles are the positively charged particles.
- Alpha particles have the least penetration power. They cannot penetrate the skin but this does not mean that they are not dangerous.
- Since they have a great ionisation power, so if they get into the body they can cause serious damage. They have the ability of ionising numerous atoms a short distance. It is due to this reason that the radioactive substance that releases alpha particles needs to be handled with rubber gloves. It should not be inhaled, eaten or allowed near open cuts.
Properties of Beta Rays.
- Beta particles are highly energetic electrons which are released from inside of a nucleus.
- They are negatively charged and have a negligible mass.
- Beta particles have a greater penetration power than the alpha particles and can easily travel through the skin.
- Though beta particles have less ionisation power than the alpha particles but still they are dangerous and so their contact with the body must be avoided.
Properties of Gamma Rays
- They have greatest power of penetration.
- They are the least ionizing but most penetrating and it is extremely difficult to stop them from entering the body.
- These rays carry huge amount of energy and can even travel through thin lead and thick concrete.
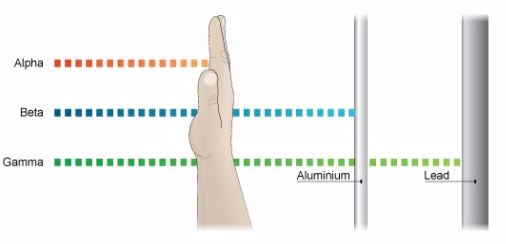
The Detection of α,β andγ Radiations
Explain the detection of α,β andγ radiations
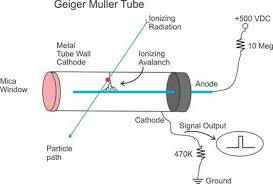
Geiger Counter, with Geiger-Mueller (GM) Tube or Probe
A GM tube is a gas-filled device that, when a high voltage is applied, creates an electrical pulse when radiation interacts with the wall or gas in the tube. These pulses are converted to a reading on the instrument meter.
If the instrument has a speaker, the pulses also give an audible click. Common readout units are roentgens per hour (R/ hr), milliroentgens per hour (mR/hr), rem per hour (rem/hr), millirem per hour (mrem/hr), and counts per minute (cpm).
GM probes (e.g., "pancake" type) are most often used with handheld radiation survey instruments for contamination measurements. However, energy-compensated GM tubes may be employed for exposure measurements.
Further, often the meters used with a GM probe will also accommodate other radiation-detection probes. For example, a zinc sulfide (ZnS) scintillator probe, which is sensitive to just alpha radiation, is often used for field measurements where alpha-emitting radioactive materials need to be measured.
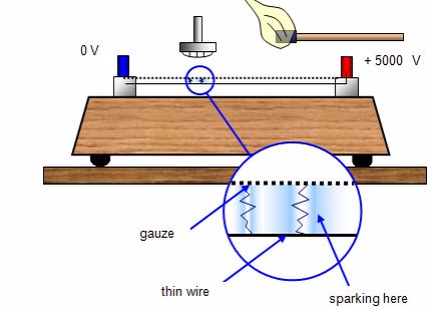
Spark counter
This consists of a fine metal gauze mounted about a millimetre away from a thin wire.A voltage is applied between the two so that sparking takes place between them - this usually requires some 4000 - 5000 V. The voltage is then reduced until sparking just stops.
If an alpha-source is brought up close to the gauze it will ionise the air, and sparks will occur between the gauze and wire. With beta and gamma sources insufficient ions are usually produced for sparking to take place.The spark counter can be used to measure the range of alpha-particles.
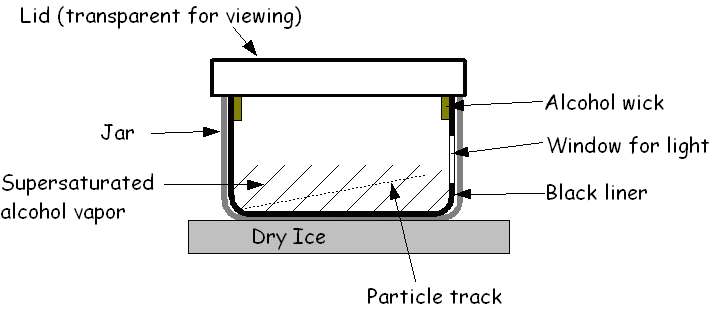
Cloud chamber
The cloud chamber, also known as the Wilson chamber, is a particle detector used for detecting ionising radiation.
Rare picture shows in a single shot the 4 particles that we can detect in a cloud chamber: proton, electron, muon (probably) and alpha. In its most basic form, a cloud chamber is a sealed environment containing a supersaturated vapor of water or alcohol.
When a charged particle (for example, an alpha or beta particle) interacts with the mixture, the fluid is ionized. The resulting ions act as condensation nuclei, around which a mist will form (because the mixture is on the point of condensation).
The high energies of alpha and beta particles mean that a trail is left, due to many ions being produced along the path of the charged particle. These tracks have distinctive shapes (for example, an alpha particle's track is broad and shows more evidence of deflection by collisions, while an electron's is thinner and straight).
When any uniform magnetic field is applied across the cloud chamber, positively and negatively charged particles will curve in opposite directions, according to the Lorentz force law with two particles of opposite charge.
Other devices used to detect radiation include:
- Photographic film
- Bubble chamber
- Gold-leaf electroscope
Half-Life as Applied to a Radioactive Substance
Describe half-life as applied to a radioactive substance
Half life can be defined as the time taken for the number of nuclei in a radioactive material to halve. It can also be defined as the time taken for the count rate of a sample of radioactive material to fall to half of its starting level. It is simply the time taken for the radioactive material to decay by half.
The count rate is measured by using an instrument called a Geiger-Muller tube over a period of time. A Geiger-Muller tube detects radiations by absorbing the radiation and converting it into an electrical pulse which triggers a counter and is displayed as a count rate.
The release of radiation by unstable nuclei is called radioactive decay. This process occurs naturally and cannot be influenced by chemical or physical processes.
The release of radiation is also a random event and overtime the activity of the radioactive material decreases. It is not possible to predict when an individual nucleus in a radioactive material will decay.
But it is possible to measure the time taken for half of the nuclei in a radioactive material to decay. This is called the half life of radioactive material or radioisotope.
The Half-Life of a Radioactive Element
Determine the half-life of a radioactive element
An exponential decay process can be described by any of the following three equivalent formulas:

where
- N0 is the initial quantity of the substance that will decay (this quantity may be measured in grams, moles, number of atoms, etc).
- N(t) is the quantity that still remains and has not yet decayed after a time t.
- t1⁄2 is the half-life of the decaying quantity.
- τis a positive number called the mean lifetime of the decaying quantity.
- λis a positive number called the decay constant of the decaying quantity.
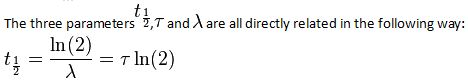
Where ln (2) is the natural logarithm of 2 (approximately 0.693).
By plugging in and manipulating these relationships, we get all of the following equivalent descriptions of exponential decay, in terms of the half-life:

The Application of a Natural Radioactive Substances
Identify the applications of a natural radioactive Substances
Medical Uses
Hospitals, doctors, and dentists use a variety of nuclear materials and procedures to diagnose, monitor, and treat a wide assortment of metabolic processes and medical conditions in humans. In fact, diagnostic x-rays or radiation therapy have been administered to about 7 out of every 10 Americans. As a result, medical procedures using radiation have saved thousands of lives through the detection and treatment of conditions ranging from hyperthyroidism to bone cancer.
The most common of these medical procedures involves the use of x-rays — a type of radiation that can pass through our skin. When x-rayed, our bones and other structures cast shadows because they are denser than our skin, and those shadows can be detected on photographic film. The effect is similar to placing a pencil behind a piece of paper and holding the pencil and paper in front of a light. The shadow of the pencil is revealed because most light has enough energy to pass through the paper, but the denser pencil stops all the light. The difference is that x-rays are invisible, so we need photographic film to "see" them for us. This allows doctors and dentists to spot broken bones and dental problems.
X-rays and other forms of radiation also have a variety of therapeutic uses. When used in this way, they are most often intended to kill cancerous tissue, reduce the size of a tumor, or reduce pain. For example, radioactive iodine (specifically iodine-131) is frequently used to treat thyroid cancer, a disease that strikes about 11,000 Americans every year.
X-ray machines have also been connected to computers in machines called computerized axial tomography (CAT) or computed tomography (CT) scanners. These instruments provide doctors with color images that show the shapes and details of internal organs. This helps physicians locate and identify tumors, size anomalies, or other physiological or functional organ problems.
In addition, hospitals and radiology centers perform approximately 10 million nuclear medicine procedures in the United States each year. In such procedures, doctors administer slightly radioactive substances to patients, which are attracted to certain internal organs such as the pancreas, kidney, thyroid, liver, or brain, to diagnose clinical conditions.
Academic and Scientific Applications
Universities, colleges, high schools, and other academic and scientific institutions use nuclear materials in course work, laboratory demonstrations, experimental research, and a variety of health physics applications. For example, just as doctors can label substances inside people's bodies, scientists can label substances that pass through plants, animals, or our world. This allows researchers to study such things as the paths that different types of air and water pollution take through the environment. Similarly, radiation has helped us learn more about the types of soil that different plants need to grow, the sizes of newly discovered oil fields, and the tracks of ocean currents.
In addition, researchers use low-energy radioactive sources in gas chromatography to identify the components of petroleum products, smog and cigarette smoke, and even complex proteins and enzymes used in medical research.
Archaeologists also use radioactive substances to determine the ages of fossils and other objects through a process called carbon dating. For example, in the upper levels of our atmosphere, cosmic rays strike nitrogen atoms and form a naturally radioactive isotope called carbon-14. Carbon is found in all living things, and a small percentage of this is carbon-14. When a plant or animal dies, it no longer takes in new carbon and the carbon-14 that it accumulated throughout its life begins the process of radioactive decay. As a result, after a few years, an old object has a lower percent of radioactivity than a newer object. By measuring this difference, archaeologists are able to determine the object's approximate age.
Industrial Uses
We could talk all day about the many and varied uses of radiation in industry and not complete the list, but a few examples illustrate the point. In irradiation, for instance, foods, medical equipment, and other substances are exposed to certain types of radiation (such as x-rays) to kill germs without harming the substance that is being disinfected and without making it radioactive. When treated in this manner, foods take much longer to spoil, and medical equipment (such as bandages, hypodermic syringes, and surgical instruments) are sterilized without being exposed to toxic chemicals or extreme heat. As a result, where we now use chlorine a chemical that is toxic and difficult-to-handle we may someday use radiation to disinfect our drinking water and kill the germs in our sewage. In fact, ultraviolet light (a form of radiation) is already used to disinfect drinking water in some homes.
Similarly, radiation is used to help remove toxic pollutants, such as exhaust gases from coal-fired power stations and industry. For example, electron beam radiation can remove dangerous sulphur dioxides and nitrogen oxides from our environment. Closer to home, many of the fabrics used to make our clothing have been irradiated (treated with radiation) before being exposed to a soil-releasing or wrinkle-resistant chemical. This treatment makes the chemicals bind to the fabric, to keep our clothing fresh and wrinkle-free all day, yet our clothing does not become radioactive. Similarly, nonstick cookware is treated with gamma rays to keep food from sticking to the metal surface.
The agricultural industry makes use of radiation to improve food production and packaging. Plant seeds, for example, have been exposed to radiation to bring about new and better types of plants. Besides making plants stronger, radiation can be used to control insect populations, thereby decreasing the use of dangerous pesticides. Radioactive material is also used in gauges that measure the thickness of eggshells to screen out thin, breakable eggs before they are packaged in egg cartons. In addition, many of our foods are packaged in polyethylene shrink-wrap that has been irradiated so that it can be heated above its usual melting point and wrapped around the foods to provide an airtight protective covering.
All around us, we see reflective signs that have been treated with radioactive tritium and phosphorescent paint. Ionizing smoke detectors, using a tiny bit of americium-241, keep watch while we sleep. Gauges containing radioisotopes measure the amount of air whipped into our ice cream, while others prevent spillover as our soda bottles are carefully filled at the factory.
Engineers also use gauges containing radioactive substances to measure the thickness of paper products, fluid levels in oil and chemical tanks, and the moisture and density of soils and material at construction sites. They also use an x-ray process, called radiography, to find otherwise imperceptible defects in metallic castings and welds. Radiography is also used to check the flow of oil in sealed engines and the rate and way that various materials wear out. Well-logging devices use a radioactive source and detection equipment to identify and record formations deep within a bore hole (or well) for oil, gas, mineral, groundwater, or geological exploration. Radioactive materials also power our dreams of outer space, as they fuel our spacecraft and supply electricity to satellites that are sent on missions to the outermost regions of our solar system.
Nuclear Power Plants
Electricity produced by nuclear fission — splitting the atom — is one of the greatest uses of radiation. As our country becomes a nation of electricity users, we need a reliable, abundant, clean, and affordable source of electricity. We depend on it to give us light, to help us groom and feed ourselves, to keep our homes and businesses running, and to power the many machines we use. As a result, we use about one-third of our energy resources to produce electricity.
Electricity can be produced in many ways — using generators powered by the sun, wind, water, coal, oil, gas, or nuclear fission. In America, nuclear power plants are the second largest source of electricity (after coal-fired plants) — producing approximately 21 percent of our Nation's electricity.
The purpose of a nuclear power plant is to boil water to produce steam to power a generator to produce electricity. While nuclear power plants have many similarities to other types of plants that generate electricity, there are some significant differences. With the exception of solar, wind, and hydroelectric plants, power plants (including those that use nuclear fission) boil water to produce steam that spins the propeller-like blades of a turbine that turns the shaft of a generator. Inside the generator, coils of wire and magnetic fields interact to create electricity. In these plants, the energy needed to boil water into steam is produced either by burning coal, oil, or gas (fossil fuels) in a furnace, or by splitting atoms of uranium in a nuclear power plant. Nothing is burned or exploded in a nuclear power plant. Rather, the uranium fuel generates heat through a process called fission.
Nuclear power plants are fueled by uranium, which emits radioactive substances. Most of these substances are trapped in uranium fuel pellets or in sealed metal fuel rods. However, small amounts of these radioactive substances (mostly gases) become mixed with the water that is used to cool the reactor. Other impurities in the water are also made radioactive as they pass through the reactor. The water that passes through a reactor is processed and filtered to remove these radioactive impurities before being returned to the environment. Nonetheless, minute quantities of radioactive gases and liquids are ultimately released to the environment under controlled and monitored conditions
The U.S. Nuclear Regulatory Commission (NRC) has established limits for the release of radioactivity from nuclear power plants. Although the effects of very low levels of radiation are difficult to detect, the NRC's limits are based on the assumption that the public's exposure to man-made sources of radiation should be only a small fraction of the exposure that people receive from natural background sources.
Experience has shown that, during normal operations, nuclear power plants typically release only a small fraction of the radiation allowed by the NRC's established limits. In fact, a person who spends a full year at the boundary of a nuclear power plant site would receive an additional radiation exposure of less than 1 percent of the radiation that everyone receives from natural background sources. This additional exposure, totaling about 1 millirem (a unit used in measuring radiation absorption and its effects), has not been shown to cause any harm to human beings.
In agriculture
Radioisotopes are used to induce mutations in plants in order to develop superior varieties that are harder and more resistant to diseases.
Artificial Radioactivity
Difference between Natural and Artificial Radioactivity
Distinguish between natural and artificial radioactivity
Artificial radioactivity is the phenomenon by which even light elements are made radioactive by artificial or induced methods.
Artificial radioactivity occurs when a previously stable material has been made radioactive by exposure to specific radiation. Most radioactivity does not induce other material to become radioactive. This Induced radioactivity was discovered by Irène Curie and F. Joliot in 1934. This is also known as man-made radioactivity. The phenomenon by which even light elements are made radioactive by artificial or induced methods is called artificial radioactivity.
Curie and Joliot showed that when lighter elements such as boron and aluminium were bombarded with α-particles, there was a continuous emission of radioactive radiations, even after the α−source had been removed. They showed that the radiation was due to the emission of a particle carrying one unit positive charge with mass equal to that of an electron.
Neutron activation is the main form of induced radioactivity, which happens when free neutrons are captured by nuclei. This new heavier isotope can be stable or unstable (radioactive) depending on the chemical element involved.
Because free neutrons disintegrate within minutes outside of an atomic nucleus, neutron radiation can be obtained only from nuclear disintegrations, nuclear reactions, and high-energy reactions (such as in cosmic radiation showers or particle accelerator collisions). Neutrons that have been slowed down through a neutron moderator (thermal neutrons) are more likely to be captured by nuclei than fast neutrons.
Methods of Producing Artificial Radioactive Isotopes
Describe methods of producing artificial radioactive isotopes
Methods of inducing radioactivity
- Nuclear activation:Neutron activation is the process in which neutron radiation induces radioactivity in materials, and occurs when atomic nuclei capture free neutrons, becoming heavier and entering excited states. The excited nucleus often decays immediately by emitting gamma rays, or particles such as beta particles, alpha particles, fission products and neutrons (in nuclear fission). Thus, the process of neutron capture, even after any intermediate decay, often results in the formation of an unstable activation product. Such radioactive nuclei can exhibit half-lives ranging from small fractions of a second to many years.
- Photonuclear reactions: A photonuclear reaction is a reaction resulting from an interaction between a photon and a nucleus.-During a photonuclear reaction energy of a gamma-ray photon is fully or partially absorbed by the nucleus forcing it into and excited state. From this excited state the nucleus can emit any particle, provided it has enough energy for such a process to occur. Most commonly it will emit a photon, but also a neutron (n), a proton (p) or an alpha (α) particle can be emitted.
Applications of Artificial Radioactivity
Mention the applications of artificial radioactivity
Application of artificial radioactivity include:
- Radiation safety:For physicians and radiation safety officers, activation of sodium in the human body to sodium-24, and phosphorus to phosphorus-32, can give a good immediate estimate of acute accidental neutron exposure.
- Neutron detection:One way to demonstrate that nuclear fusion has occurred inside a fusor device is to use a Geiger counter to measure the gamma ray radioactivity that is produced from a sheet of aluminum foil.In the ICF fusion approach, the fusion yield of the experiment (directly proportional to neutron production) is usually determined by measuring the gamma-ray emissions of aluminum or copper neutron activation targets.Aluminum can capture a neutron and generate radioactive sodium-24, which has a half-life of 15 hours[7][8] and a beta decay energy of 5.514 MeV. The activation of a number of test target elements such as sulfur, copper, tantalum and gold have been used to determine the yield of both pure fission and thermonuclear weapons.
- Materials analysis:Neutron activation analysis is one of the most sensitive and accurate methods of trace element analysis. It requires no sample preparation and can therefore be applied to objects that need to be kept intact such as a valuable piece of art. Although the activation induces radioactivity in the object, its level is typically low and its lifetime may be short, so that its effects soon disappear. In this sense, neutron activation is a non-destructive analysis method.
- The potential use of photo-nuclear reactions for a range of applications is described. These are: photo-nuclear transmutation doping of semiconductors, neutron production from electron linacs, quality checking of radioactive waste, fission product incineration, photo-excitation of isomers for dosimetry, and nuclear resonance fluorescence for materials analysis. Initial brief descriptions of atomic and nuclear interactions of photons are given.
Radiation Hazards and Safety
The Effects of Nuclear Radiation on Human Body
Explain the effects of nuclear radiation on human body
Certain body parts are more specifically affected by exposure to different types of radiation sources. Several factors are involved in determining the potential health effects of exposure to radiation. These include:
- The size of the dose (amount of energy deposited in the body)
- The ability of the radiation to harm human tissue
- Which organs are affected
The most important factor is the amount of the dose - the amount of energy actually deposited in your body. The more energy absorbed by cells, the greater the biological damage. Health physicists refer to the amount of energy absorbed by the body as the radiation dose. The absorbed dose, the amount of energy absorbed per gram of body tissue, is usually measured in units called rads. Another unit of radation is the rem, or roentgen equivalent in man. To convert rads to rems, the number of rads is multiplied by a number that reflects the potential for damage caused by a type of radiation. For beta, gamma and X-ray radiation, this number is generally one. For some neutrons, protons, or alpha particles, the number is twenty.
- Hair:The losing of hair quickly and in clumps occurs with radiation exposure at 200 rems or higher.
- Brain:Since brain cells do not reproduce, they won't be damaged directly unless the exposure is 5,000 rems or greater. Like the heart, radiation kills nerve cells and small blood vessels, and can cause seizures and immediate death.
- Thyroid:The certain body parts are more specifically affected by exposure to different types of radiation sources. The thyroid gland is susceptible to radioactive iodine. In sufficient amounts, radioactive iodine can destroy all or part of the thyroid. By taking potassium iodide can reduce the effects of exposure.
- Blood System:When a person is exposed to around 100 rems, the blood's lymphocyte cell count will be reduced, leaving the victim more susceptible to infection. This is often refered to as mild radiation sickness. Early symptoms of radiation sickness mimic those of flu and may go unnoticed unless a blood count is done. According to data from Hiroshima and Nagaski, show that symptoms may persist for up to 10 years and may also have an increased long-term risk for leukemia and lymphoma. For more information, visit Radiation Effects Research Foundation.
- Heart:Intense exposure to radioactive material at 1,000 to 5,000 rems would do immediate damage to small blood vessels and probably cause heart failure and death directly.
- Gastrointestinal Tract:Radiation damage to the intestinal tract lining will cause nausea, bloody vomiting and diarrhea. This is occurs when the victim's exposure is 200 rems or more. The radiation will begin to destroy the cells in the body that divide rapidly. These including blood, GI tract, reproductive and hair cells, and harms their DNA and RNA of surviving cells.
- Reproductive Tract:Because reproductive tract cells divide rapidly, these areas of the body can be damaged at rem levels as low as 200. Long-term, some radiation sickness victims will become sterile.
Radiation sickness
Radiation sickness results when humans (or other animals) are exposed to very large doses of ionizing radiation. Radiation exposure can occur as a single large exposure (acute), or a series of small exposures spread over time (chronic). Exposure may be accidental or intentional (as in radiation therapy).
Causes
- Accidental exposure to high doses of radiation such as a nuclear power plant accidents.
- Exposure to excessive radiation for medical treatments.
Symptoms
- Bleeding from the nose, mouth, gums, and rectum
- Bloody stool
- Bruising
- Confusion
- Dehydration
- Diarrhea
- Fainting
- Fatigue
- Fever
- Hair loss
- Inflammation of exposed areas (redness, tenderness, swelling, bleeding)
- Mouth ulcers
- Nausea and vomiting
- Open sores on the skin
- Skin burns (redness, blistering)
- Sloughing of skin
- Ulcers in the esophagus, stomach or intestines
- Vomiting blood
- Weakness
First Aid
- Check the person's breathing and pulse.
- Start CPR, if necessary.
- Remove the person's clothing and place the items in a sealed container. This stops ongoing contamination.
- Vigorously wash body with soap and water.
- Dry the body and wrap with soft, clean blanket.
- Call for emergency medical help or take the person to nearest emergency medical facility if you can do so safely.
How to Protect yourself from Nuclear Radiation Hazards
Protect himself/herself from nuclear radiation hazards
Precautions
- Time:An average the procedure time for a diagnostic coronary angiogram is approximately 30 minutes and an interventional procedure PCI or EPS/pacing would take between 90 to 120 minutes. However the fluoroscopic and the cine screening time are highly variable depending on the nature of the procedure and the experience of the operator. The lower the amount of time spent in a radiation area, the lower the exposure will be. Significant reductions can be achieved when an activity is delayed until after cine imaging is completed. Every effort should be made by the operating cardiologist in the cath lab to minimise fluoroscopy and cine screening time.
- Distance:Increasing the distance from the radiation beam decreases the risk of exposure. doubling the distance between the primary beam and operator, reduces the exposure by a factor of four. In addition, the radiation exposure varies according to the angle at which the camera is projected Oblique views (left and right anterior oblique) and steep angulations increase radiation exposure but are often employed to improve visualisation. 60-degree angulations give up to three times the operator dose than 30-degree angulations (11). The second operator or assistant is generally less exposed to radiation compared to the first operator but certainly more at risk than the other staff in the room.
- Shielding:Lead shields and shielding will significantly reduce the risk of exposure but only if appropriately used and in proper working order. Protective equipment includes lead aprons, thyroid collars and leaded glasses. With the newly designed frames and ultra light lenses, protective leaded eyewear is now used by more of the cardiologists and staff in cardiac cath lab. Some cath labs also use overhanging lead screens to prevent radiation exposure to brain. The staff should wear a protective apron of at least 0.25 mm lead equivalent. Protective gloves should be of at least 0.35 mm lead equivalent. All such protective clothing should bear an identifying mark and should be examined at yearly intervals. Defective items should be withdrawn from use.
- Adhering to guideline and protocols:Every unit or work place that deals with ionising radiation should have their own local guidelines and rules for radiation safety. These must be read, understood and strictly adhered to in daily practice. Staff must comply with these local rules in order to insure that the Trust and all their employees do not contravene statutory requirements of the ionising radiation regulations and other relevant legislation.
- Minimising risk of exposure to staff and patients: The occupational limit of radiation exposure in the UK currently is estimated at 20 mSv per year averaged over five consecutive years (5). Every operator who undertakes a cardiovascular procedure in the cath lab is responsible for the amount of radiation exposure to the patient, his or her co-staff and to themselves. In the event of an incident where the patient might have been exposed to inadvertent excess radiation either due to clinical circumstances, malfunctioning of the equipment or operation errors, the radiation protection adviser should be informed of the incident. It is their duty to estimate the radiation dose received by the patient and also advise whether the incident is to be reported.Only essential staff shall be in the cath lab during radiation exposure. All persons not required in the room should leave the room during serial radiographic exposure. The operator shall stand behind a barrier if possible. People who must move around the room during the procedure should wear a wraparound protective garment. When possible, the cardiologist and all other personnel required in the room should step back from the table and behind portable shields during cine and serial radiography procedures. This action can decrease the exposure of the cardiologist and the other nearby personnel by a factor of three or more (10).
Nuclear Fission and Fusion
The Nuclear Fission and Fusion
Explain the nuclear fission and fusion
Nuclear fission
Nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei).
The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place).
In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.
Nuclear fusion
Nuclear fusion is a nuclear reaction in which two or more atomic nuclei come very close and then collide at a very high speed and join to form a new type of atomic nucleus.
During this process, matter is not conserved because some of the matter of the fusing nuclei is converted to photons (energy).
The fusion of two nuclei with lower masses than iron (which, along with nickel, has the largest binding energy per nucleon) generally releases energy, while the fusion of nuclei heavier than iron absorbs energy.
The opposite is true for the reverse process, nuclear fission. This means that fusion generally occurs for lighter elements only, and likewise, that fission normally occurs only for heavier elements.







No comments:
Post a Comment
Note: Only a member of this blog may post a comment.